Entitlement Eligibility Guideline (EEG)
Date created: 22 January 2025
ICD-11 code: AB31.0
VAC medical code: 38600 Meniere’s disease
This publication is available upon request in alternate formats.
Full document – PDF Version
Definition
Meniere’s disease (MD) is a disorder of the inner ear characterized by spontaneous, paroxysmal attacks of vertigo, hearing loss (HL), tinnitus, and a sense of fullness in the involved ear.
For this entitlement eligibility guideline (EEG), equivalent diagnoses for Meniere’s disease include:
- Meniere’s syndrome
- endolymphatic hydrops.
For Veterans Affairs Canada (VAC) purposes, vertigo, HL, and/or tinnitus may present as part of the symptom complex of a diagnosed medical condition, or they may present as a primary stand-alone diagnosed medical condition. In those presenting with symptoms of vertigo, HL, and/or tinnitus, but with a known diagnosed cause (e.g. Meniere’s disease), these symptoms are included in entitlement and assessment of the medical condition. Prior to adjudicating the entitlement and assessment of vertigo, HL, and/or tinnitus, or a diagnosed medical condition that may cause these symptoms, a close review of previously entitled medical conditions with potentially overlapping symptoms is required.
Diagnostic standard
A diagnosis of MD by a qualified physician (ear, nose and throat [ENT] specialist/otolaryngologist or neurologist) is required. There is no specific diagnostic test for MD and the diagnosis is one of exclusion, meaning the diagnosis can only be made when no other cause is found for the symptoms.
The diagnosis of MD is definite when the following criteria are met:
- Two or more spontaneous attacks of vertigo with spontaneous onset, each lasting 20 minutes to 12 hours.
- Audiometrically documented fluctuating sensorineural HL on at least one occasion before, during, or after one of the episodes of vertigo. The HL is in the low to mid frequencies and unilateral.
- Tinnitus and/or aural fullness in the affected ear.
- No other cause is likely.
The diagnosis of MD is probable when the following criteria are met:
- at least two episodes of vertigo or dizziness lasting 20 minutes to 24 hours
- fluctuating aural symptoms (HL, tinnitus, or fullness), in the affected ear
- no other cause is likely.
Note: For VAC entitlement purposes, only a definite diagnosis of MD can be entitled; probable MD is not acceptable for entitlement purposes.
Current investigations should include an audiogram. Investigations may also include, but are not limited to, magnetic resonance imaging (MRI) and computed tomography (CT) scans. Reports should accompany the application.
The diagnosis of MD is a clinical one. The attacks are random, episodic, and may have long gaps with no symptoms. The diagnosis may take years of symptoms, typically at least three to five years, and requires first ruling out other causes for the symptoms.
Historically, MD has been classified by the following definitions:
- Meniere’s disease (MD) versus Meniere’s syndrome (MS): These two diagnoses have in the past been distinguished by the presence, or absence, of a known cause where MD was considered to be an idiopathic condition and MS had a known cause. The diagnostic distinction between MD and MS is usually not considered at this time because, by the time the symptoms arise, the ear has already been damaged and the cause may not be obvious.
- Atypical versus typical MD: These diagnostic distinctions have been used to identify the exact site of dysfunction within the inner ear. There may be a further breakdown of atypical MD into vestibular versus cochlear symptoms.
Currently, and for the purposes of this EEG, the diagnosis of MD is based on symptoms and the diagnostic criteria. All types or classifications of MD are included and the diagnostic terms, both old and new, are equivalent.
Anatomy and physiology
The pathophysiology of MD is poorly understood. The classic pathologic lesion of MD is endolymphatic hydrops (EH), which is an increase in inner ear fluid volumes (Figure 1: Ear anatomy). However, while EH is present in all those with MD, not everyone with EH is symptomatic.
Figure 1: Ear anatomy
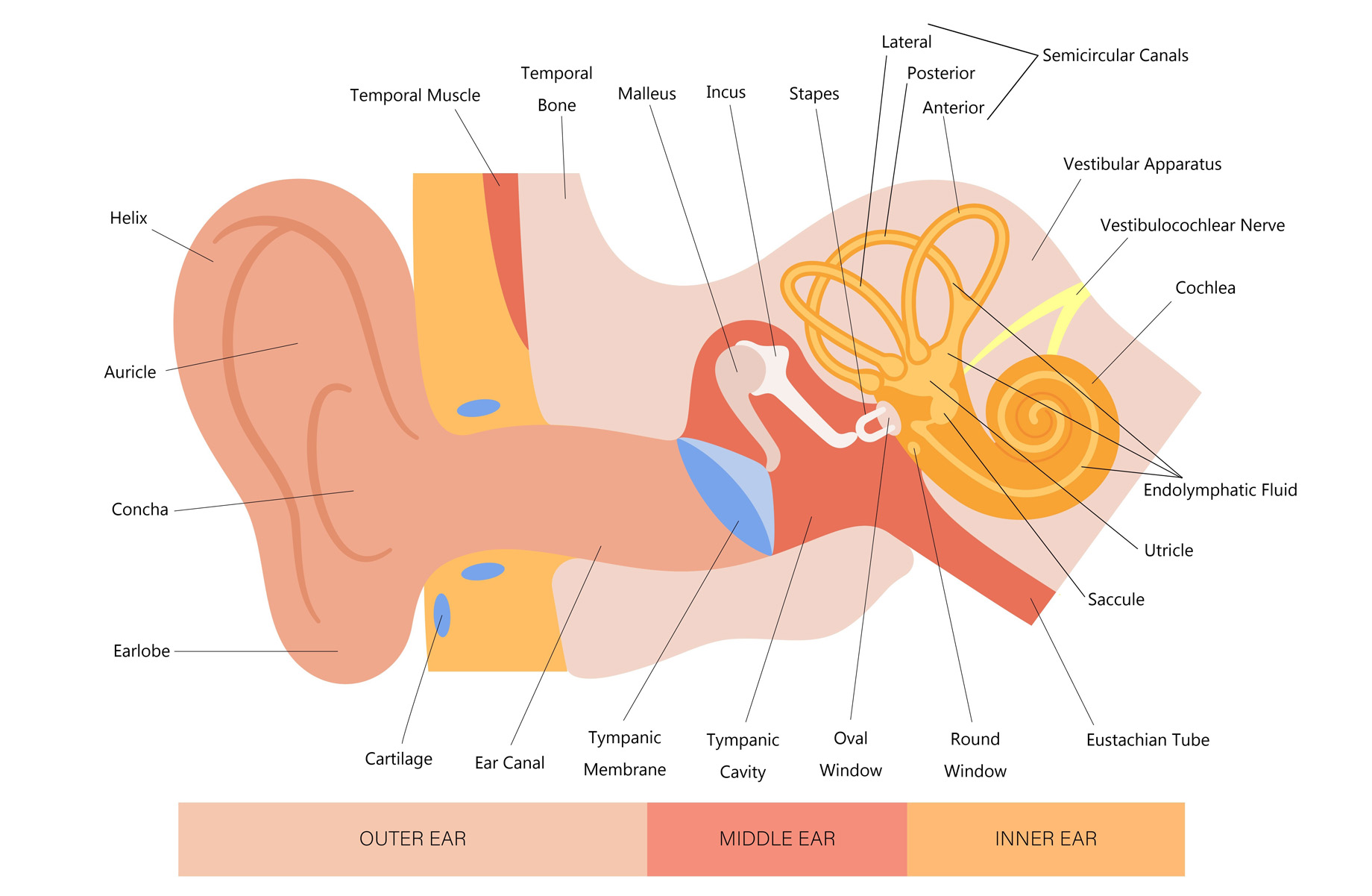
An illustration of the human ear, clearly labeling its three main sections and associated structures. Outer ear: Includes visible parts such as the helix, auricle, concha, earlobe, and external ear canal. Middle ear: Contains the tympanic membrane (eardrum), three small bones called ossicles (malleus, incus, stapes), and the eustachian tube. Inner ear: Features the semicircular canals, cochlea, utricle and saccule – all filled with endolymphatic fluid. Associated nerves are the cochlear and vestibular nerves. Between the middle and inner ear is the oval window and the round window. Source: Veterans Affairs Canada (2024).
EH can result from multiple causes. While some evidence of EH may be found on MRI and CT scan, the diagnosis remains clinical at this time. MRI and CT are performed to indicate other causes for the symptoms.
The literature indicates a multifactorial basis for all forms of MD, with the fundamental problem appearing to be endolymphatic malabsorptive dysfunction, suggestive of a combination of multiple genetic and non-genetic, or environmental factors.
Clinical features
The average age of onset of MD is in the fifth decade of life, although symptoms typically begin between 40 and 60 years of age. MD may affect one or both ears. For VAC entitlement and assessment purposes, it is considered a bilateral condition.
MD consists of a triad of symptoms of vertigo, HL, and tinnitus. These symptoms may be experienced at separate times, with some occurring months to years before others. The diagnosis cannot be definitively made until all symptoms are present and other causes for the symptoms are ruled out. MD usually presents as acute episodes of vertigo lasting from a few minutes to several hours, followed by periods of remission. There may be weeks or months between acute episodes, or they may increase in severity and frequency so that a person is effectively incapacitated during repeated episodes of vertigo and nausea.
Vertigo is typically rotatory (spinning) and lasts 20 minutes to 24 hours. There may be associated nausea and vomiting. Most people have resolution of their vertigo symptoms through treatment.
The type of hearing loss associated with MD is recurrent low frequency HL; this type of HL has causes other than MD. The HL initially is fluctuating, typically sensorineural and in the lower frequencies. HL is usually associated with fullness in the affected ear. The HL usually worsens over time, spreading over all frequencies, and often becomes permanent over 8-10 years, regardless of treatment.
Tinnitus is fluctuating in both pitch and intensity. It can occur with or without vertigo or HL.
Most commonly, MD presents with episodes of vertigo; however, HL or tinnitus may be the initial symptom. For VAC entitlement purposes, the onset of all symptoms should occur within a five-year time frame.
There is a slight increased incidence (1.3-1.89:1) of MD in females as compared to males.
Entitlement considerations
In this section
Section A: Causes and/or aggravation
Section B: Medical conditions which are to be included in entitlement/assessment
Section A: Causes and/or aggravation
For VAC entitlement purposes, the following factors are accepted to cause or aggravate the conditions included in the Definition section of this EEG, and may be considered along with the evidence to assist in establishing a relationship to service. The factors have been determined based on a review of up-to-date scientific and medical literature, as well as evidence-based medical best practices. Factors other than those listed may be considered, however consultation with a disability consultant or medical advisor is recommended.
The timelines cited below are for guidance purposes. Each case should be adjudicated on the evidence provided and its own merits.
Factors
- MD is of idiopathic (unknown) origin.
- Inability to obtain appropriate clinical management of MD.
Note: At the time of publication, the health-related expert opinion and scientific evidence indicates the following:
- noise induced HL does not cause MD
- acoustic trauma does not cause MD.
Section B: Medical conditions which are to be included in entitlement/assessment
Section B provides a list of diagnosed medical conditions which are considered for VAC purposes to be included in the entitlement and assessment of MD.
Section C: Common medical conditions which may result, in whole or in part, from Meniere’s disease and/or its treatment
No consequential medical conditions were identified at the time of the publication of this EEG. If the merits of the case and medical evidence indicate that a possible consequential relationship may exist, consultation with a disability consultant or medical advisor is recommended.
Links
Related VAC guidance and policy:
- Hearing Loss – Entitlement Eligibility Guidelines
- Tinnitus – Entitlement Eligibility Guidelines
- Vertiginous Disorders – Entitlement Eligibility Guidelines
- Pain and Suffering Compensation – Policies
- Royal Canadian Mounted Police Disability Pension Claims – Policies
- Dual Entitlement – Disability Benefits – Policies
- Establishing the Existence of a Disability – Policies
- Disability Benefits in Respect of Peacetime Military Service – The Compensation Principle – Policies
- Disability Benefits in Respect of Wartime and Special Duty Service – The Insurance Principle – Policies
- Disability Resulting from a Non-Service Related Injury or Disease – Policies
- Consequential Disability – Policies
- Benefit of Doubt – Policies
References as of 22 January 2025
Angum, F., Khan, T., Kaler, J., Siddiqui, L., & Hussain, A. (2020). The Prevalence of Autoimmune Disorders in Women: A Narrative Review. Cureus. https://doi.org/10.7759/cureus.8094
Australian Government, Repatriation Medical Authority. (2015). Statement of Principles concerning Meniere’s disease (Balance of Probabilities) (No. 108 of 2015). SOPs - Repatriation Medical Authority
Australian Government, Repatriation Medical Authority. (2015). Statement of Principles concerning Meniere’s disease (Reasonable Hypothesis) (No. 109 of 2015). SOPs - Repatriation Medical Authority
Basura, G. J., Adams, M. E., Monfared, A., Schwartz, S. R., Antonelli, P. J., Burkard, R., Bush, M. L., Bykowski, J., Colandrea, M., Derebery, J., Kelly, E. A., Kerber, K. A., Koopman, C. F., Kuch, A. A., Marcolini, E., McKinnon, B. J., Ruckenstein, M. J., Valenzuela, C. V., Vosooney, A., … Buchanan, E. M. (2020). Clinical Practice Guideline: Ménière’s Disease Executive Summary. Otolaryngology–Head and Neck Surgery, 162(4), 415–434. https://doi.org/10.1177/0194599820909439
Berkow, R., Fletcher, A. J., & Bondy, P. K (1992). The Merck manual of diagnosis and therapy (16th ed.). Merck Research Laboratories; WorldCat.
Borsetto, D., Corazzi, V., Obholzer, R., Bianchini, C., Pelucchi, S., Solmi, M., Jiang, D., Amin, N., Pai, I., & Ciorba, A. (2023). Dizziness, psychological disorders and cognitive decline. Panminerva Medica, 65(1). https://doi.org/10.23736/S0031-0808.21.04209-9
Caruso, S., Mauro, D., Maiolino, L., Grillo, C., Rapisarda, A. M. C., & Cianci, S. (2018). Effects of combined oral contraception containing drospirenone on premenstrual exacerbation of Meniere’s disease: Preliminary study. European Journal of Obstetrics & Gynecology and Reproductive Biology, 224, 102–107. https://doi.org/10.1016/j.ejogrb.2018.03.015
Caulley, L., Quimby, A., Karsh, J., Ahrari, A., Tse, D., & Kontorinis, G. (2018). Autoimmune arthritis in Ménière’s disease: A systematic review of the literature. Seminars in Arthritis and Rheumatism, 48(1), 141–147. https://doi.org/10.1016/j.semarthrit.2017.11.008
Chen, Y., Zhao, P., Ma, X., Diao, T., & Yu, L. (2023). Case report: MRI changes of the inner ear in an MD patient with suspected immune dysfunction. Frontiers in Neurology, 14, 1220162. https://doi.org/10.3389/fneur.2023.1220162
Fauci, A., Braunwald, E., Isselbacher, K., Wilson, J., Martin, J., Kasper, D., Hauser, S., & Longo, D. (1998). Harrison’s Principles of Internal Medicine 14th Edition. 36(9), 665–665. https://doi.org/10.1038/sj.sc.3100671
Gazquez, I., Soto-Varela, A., Aran, I., Santos, S., Batuecas, A., Trinidad, G., Perez-Garrigues, H., Gonzalez-Oller, C., Acosta, L., & Lopez-Escamez, J. A. (2011). High Prevalence of Systemic Autoimmune Diseases in Patients with Menière’s Disease. PLoS ONE, 6(10), e26759. https://doi.org/10.1371/journal.pone.0026759
Harrison, T. R., & Fauci, A. S. (Eds.). (1998). Harrison’s principles of internal medicine (14th ed.). McGraw-Hill, Health Professions Division.
Huppert, D., Strupp, M., & Brandt, T. (2010). Long-term course of Menière’s disease revisited. Acta Oto-Laryngologica, 130(6), 644–651. https://doi.org/10.3109/00016480903382808
Izmirly, P. M., Parton, H., Wang, L., McCune, W. J., Lim, S. S., Drenkard, C., Ferucci, E. D., Dall’Era, M., Gordon, C., Helmick, C. G., & Somers, E. C. (2021). Prevalence of Systemic Lupus Erythematosus in the United States: Estimates From a Meta‐Analysis of the Centers for Disease Control and Prevention National Lupus Registries. Arthritis & Rheumatology, 73(6), 991–996. https://doi.org/10.1002/art.41632
Jian, H., Yu, G., Chen, G., Lin, N., & Wang, H. (2019). Correlation between auditory‐vestibular functions and estrogen levels in postmenopausal patients with Meniere’s disease. Journal of Clinical Laboratory Analysis, 33(1), e22626. https://doi.org/10.1002/jcla.22626
Kim, S. Y., Song, Y. S., Wee, J. H., Min, C., Yoo, D. M., & Choi, H. G. (2020). Association between Ménière’s disease and thyroid diseases: A nested case–control study. Scientific Reports, 10(1), 18224. https://doi.org/10.1038/s41598-020-75404-y
Kurtzman, J. S., & Sioshansi, P. C. (2023). Infectious causes and mimickers of Meniere’s disease. Current Opinion in Otolaryngology & Head & Neck Surgery, 31(5), 332–339. https://doi.org/10.1097/MOO.0000000000000909
Loureiro, R. M., Sumi, D. V., Tames, H. L. de V. C., Soares, C. R., Salmito, M. C., Gomes, R. L. E., & Daniel, M. M. (2020). Endolymphatic hydrops evaluation on MRI: Practical considerations. American Journal of Otolaryngology, 41(2), 102361. https://doi.org/10.1016/j.amjoto.2019.102361
Mammen, J. S. R., & Cappola, A. R. (2021). Autoimmune Thyroid Disease in Women. JAMA, 325(23), 2392. https://doi.org/10.1001/jama.2020.22196
Mohamed, S., Khan, I., Iliodromiti, S., Gaggini, M., & Kontorinis, G. (2016). Ménière’s Disease and Underlying Medical and Mental Conditions: Towards Factors Contributing to the Disease. ORL, 78(3), 144–150. https://doi.org/10.1159/000444931
Morse, G. G., & House, J. W. (n.d.). Changes in Ménière’s Disease Responses as a Function of the Menstrual Cycle.
Moskowitz, H. (2022). Meniere disease: Evaluation, diagnosis, and management. UpToDate, Inc.
Mulder, J. E. (1998). THYROID DISEASE IN WOMEN. Medical Clinics of North America, 82(1), 103–125. https://doi.org/10.1016/S0025-7125(05)70596-4
Newby, H. A., & Popelka, G. R. (1992). Audiology (6th ed.). Prentice Hall; WorldCat.
Orji, F. (2014). The influence of psychological factors in Meniere′s disease. Annals of Medical and Health Sciences Research, 4(1), 3.
Paparella, M. M., da Costa, S. S., & Fagan, J. (1991). Paparella’s Otolaryngology: Head & Neck Surgery: Two Volume Set (3rd ed.). Jaypee Brothers Medical Publishers.
Perez-Carpena, P., & Lopez-Escamez, J. A. (2020). Current Understanding and Clinical Management of Meniere’s Disease: A Systematic Review. Seminars in Neurology, 40(01), 138–150. https://doi.org/10.1055/s-0039-3402065
Pieskä, T., Kotimäki, J., Männikkö, M., Sorri, M., & Hietikko, E. (2018). Concomitant diseases and their effect on disease prognosis in Meniere’s disease: Diabetes mellitus identified as a negative prognostic factor. Acta Oto-Laryngologica, 138(1), 36–40. https://doi.org/10.1080/00016489.2017.1373850
Qin, B., Wang, J., Yang, Z., Yang, M., Ma, N., Huang, F., & Zhong, R. (2015). Epidemiology of primary Sjögren’s syndrome: A systematic review and meta-analysis. Annals of the Rheumatic Diseases, 74(11), 1983–1989. https://doi.org/10.1136/annrheumdis-2014-205375
Quintero, O. L., Amador-Patarroyo, M. J., Montoya-Ortiz, G., Rojas-Villarraga, A., & Anaya, J.-M. (2012). Autoimmune disease and gender: Plausible mechanisms for the female predominance of autoimmunity. Journal of Autoimmunity, 38(2–3), J109–J119. https://doi.org/10.1016/j.jaut.2011.10.003
Radtke, A., Lempert, T., Gresty, M. A., Brookes, G. B., Bronstein, A. M., & Neuhauser, H. (n.d.). Migraine and Ménière’s disease.
Rees, F., Doherty, M., Grainge, M. J., Lanyon, P., & Zhang, W. (2017). The worldwide incidence and prevalence of systemic lupus erythematosus: A systematic review of epidemiological studies. Rheumatology, 56(11), 1945–1961. https://doi.org/10.1093/rheumatology/kex260
Rizk, H. G., Mehta, N. K., Qureshi, U., Yuen, E., Zhang, K., Nkrumah, Y., Lambert, P. R., Liu, Y. F., McRackan, T. R., Nguyen, S. A., & Meyer, T. A. (2022). Pathogenesis and Etiology of Ménière Disease: A Scoping Review of a Century of Evidence. JAMA Otolaryngology–Head & Neck Surgery, 148(4), 360. https://doi.org/10.1001/jamaoto.2021.4282
Saeed, S. R. (1998). Fortnightly review: Diagnosis and treatment of Meniere’s disease. BMJ, 316(7128), 368–372. https://doi.org/10.1136/bmj.316.7128.368
Schwartz, H. (2023). Meniere Disease. In DynaMed. EBSCO Information Services. https://www.dynamed.com/condition/Meniere-disease/about
Segal, S., Eviatar, E., Berenholz, L., Kessler, A., & Shlamkovitch, N. (2003). Is There a Relation Between Acoustic Trauma or Noise-Induced Hearing Loss and a Subsequent Appearance of Ménière’s Disease?: An Epidemiologic Study of 17,245 Cases and a Review of the Literature: Otology & Neurotology, 24(3), 387–391. https://doi.org/10.1097/00129492-200305000-00007
Somers, E. C., Marder, W., Cagnoli, P., Lewis, E. E., DeGuire, P., Gordon, C., Helmick, C. G., Wang, L., Wing, J. J., Dhar, J. P., Leisen, J., Shaltis, D., & McCune, W. J. (2014). Population‐Based Incidence and Prevalence of Systemic Lupus Erythematosus: The Michigan Lupus Epidemiology and Surveillance Program. Arthritis & Rheumatology, 66(2), 369–378. https://doi.org/10.1002/art.38238
Stölzel, K., Droste, J., Voß, L. J., Olze, H., & Szczepek, A. J. (2018). Comorbid Symptoms Occurring During Acute Low-Tone Hearing Loss (AHLH) as Potential Predictors of Menière’s Disease. Frontiers in Neurology, 9, 884. https://doi.org/10.3389/fneur.2018.00884
Thompson, T. L., & Amedee, R. (2009). Vertigo: A review of common peripheral and central vestibular disorders. Ochsner Journal, 9(1), 20–26.
Tyrrell, J., White, M. P., Barrett, G., Ronan, N., Phoenix, C., Whinney, D. J., & Osborne, N. J. (2015). Mental Health and Subjective Well-being of Individuals With Ménière’s: Cross-sectional Analysis in the UK Biobank. Otology & Neurotology, 36(5), 854–861. https://doi.org/10.1097/MAO.0000000000000732
Veterans Affairs Canada (2024). Ear Anatomy. License purchased for use from Ear Anatomy Diagram Royalty Free SVG, Cliparts, Vectors, and Stock Illustration. Image 173564185. (123rf.com)
Whitacre, C. C. (2001). Sex differences in autoimmune disease. Nature Immunology, 2(9), 777–780. https://doi.org/10.1038/ni0901-777
World Health Organization. (2019). International statistical classification of diseases and related health problems (11th Revision). https://icd.who.int/
Wu, K., Wang, D., Wang, H., Wang, H., Guan, J., Zhao, L., & Wang, Q. (2020). [Diagnosis and outcome analysis of diseases characterized by recurrent low-frequency sensorineural deafness]. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi = Journal of Clinical Otorhinolaryngology, Head, and Neck Surgery, 34(2), 106–112.